Phenotypic Data
Data Overview |
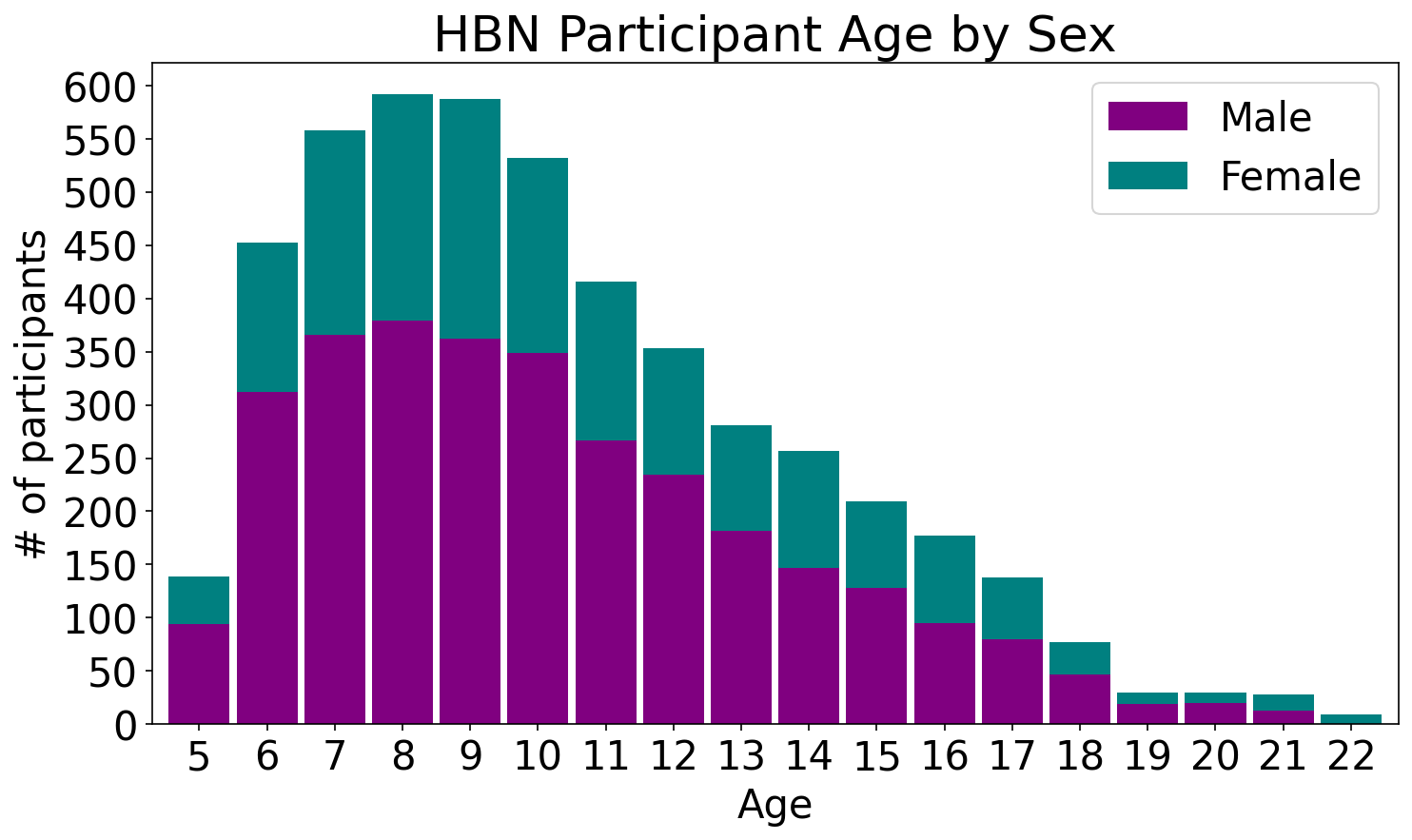
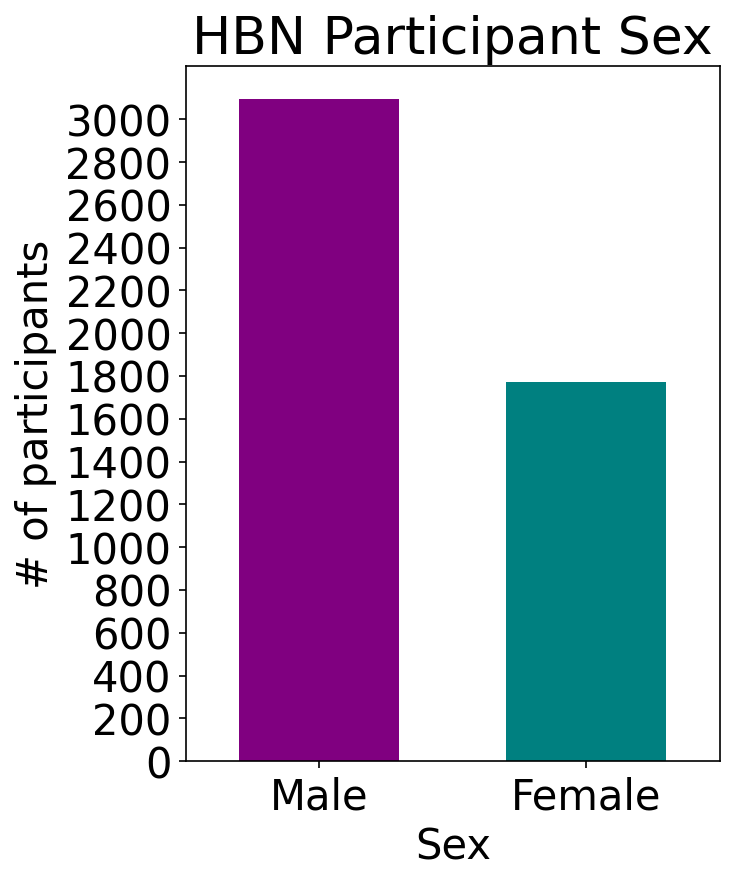
Sample Characteristics |
Data Usage Agreement |
Accessing Phenotypic Data |
Diagnostic Process |
|---|
☰
Data Overview
Phenotypic Protocol
Domains:- Demographics
- Cognition
- Language
- Emotional and Psychological Function
- Social, Emotional, and Behavioral Functioning
- Family Structure, Stress, and Trauma
- Physical Skills, Fitness, and Physiologic Status
- Substance Use and Addiction
- Neurologic Function and Eye-movements
- Medical Status
- Diagnosis
Phenotypic data may be accessed through an HBN-dedicated instance of the Longitudinal Online Research and Imaging System (LORIS) or the COllaborative Informatics and Neuroimaging Suite (COINS) Data Exchange.
Click here to download a zip folder with all data dictionaries for Release 11.
Prior to entering clinical data into these data sharing tools, all personal identifiers defined as Protected Health Information (PHI) by the Health Insurance Portability and Accountability Act (HIPAA) are removed. Consistent with standard FCP/INDI protocols, users receive meta-data as comma-separated files (.csv) which does not contain any participant PHI. For each participant, a unique random identifier is used to label all data. During the consent process, all participants provide informed consent for their data to be openly shared following this de-identification process. Please note that not all participants with imaging data will have had phenotypic data released at the same time.
Electronic Data
Electronic data is collected via NextGen. NextGen provides the infrastructure for both the collection of applicable phenotypic assessments and the participant portal. All data collected electronically is stricken of any participant protected health information (PHI). Participants are given a random unique identifier, generated directly from the electronic data capture system or the Global Unique Identified (GUID) Tool at the start of participation. The GUID Tool is a customized software application that generates a Global Unique Identifier for each study participant. The GUID is made up of random alpha-numeric characters and is NOT generated from PHI. As such, it has been approved by the NIH Office of General Counsel. All data are labeled with this unique identifier. Applicable participants receive a unique login and password to enter on the Healthy Brain Networks participant portal that serves as a secure, HIPAA-compliant, easy way for participants, and parents of participants, to complete relevant study measures assessments that are not collected during the face-to-face session. The Participant Portal is equipped with a variety of security features, including 128-bit encryption, firewall, anti-virus software, and intrusion detection software. Messaging features are also secure. All electronic data collected as part of the HBN initiative is kept on password- protected computers. Only approved researchers will be granted with logins for electronic data entry, review, and processing.
Paper Records
Paper records, including consent, assent, and HIPAA forms, and paper-CRFs are kept in participant files in locked file cabinets. These materials are additional kept behind locked doors. Participant data collected via paper-CRFs will not contain any participant PHI. These data forms will be de-identified and contain only the participant’s unique identifier. Additionally, data collected via paper are inputted into the electronic data system. Therefore all participant data are contained within a single, secure, electronic system to facilitate data sharing efforts of the project.
Participant Privacy
Confidentiality was a paramount consideration in planning data-sharing requirements. Protecting participant privacy while also providing access to extensively revealing data was a goal. All imaging data are fully anonymized in compliance with HIPAA by removing any potential protected health information identifiers, including identifying facial features from anatomical images, and randomizing the timing of release. It is important to note that data users must be aware of the possible negative impact of defacing on some analysis toolkits (e.g., FreeSurfer), and exercise additional care when producing such images and/or sharing pre-processed surfaces.
Data Usage Agreement
To access the HBN phenotypic dataset you need to complete a Data Usage Agreement (DUA):
- The adoption of a data usage agreement is not intended to limit the specific analyses a researcher can perform. The intent of the agreement is to ensure that data users agree to protect participant confidentiality when handling data that contains potentially identifying information and that they will agree to take the necessary measures to prevent breaches of privacy.
- The specific agreement to be employed for the HBN Dataset are those previously defined by the New York State Office of Mental Health. Institutional review board (IRB) approval is not required for transfer of the data; it will be up to the individual data user to satisfy any additional requirements specified by their local IRB or ethics committee, prior to using the HBN Dataset. Given that local IRB approval is not required as part of an individuals application for access to the HBN Dataset, there is no need for an individual’s IRB to have a federal-wise assurance number.
- Complete the DUA form at https://redcap.link/HBN_DUA, which includes instructions on how to download and complete the DUA.
- Make sure you have reviewed all instructions and DUA contents. Edits to the DUA are not permitted. Note that DUAs can take up to 3 weeks to be processed and approved.
- When the DUA application has been received and approved, you will receive an email providing you access to all phenotypic data in the LORIS Database.
Accessing Phenotypic Data
When your DUA application has been received and approved, you may access the full phenotypical data through LORIS. Click here to find a comprehensive list of assessments in LORIS. Data can be also found on COINS (you can find instructions on how to access data in COINS here) however, since 2022, we are no longer maintaining the dataset on that platform.
LORIS
LORIS (Longitudinal Online Research and Imaging System) is a web-based data and project management software for neuroimaging research studies.
LORIS is designed to simplify integration, management, and dissemination of cohort studies that involve various neuroimaging modalities, behavioral tests, and genetic and neurophysiological data. It provides secure web-based access to data validation and quality control modules, as well as visualization and basic statistical tools. LORIS is in compliance with Health Insurance Portability and Accountability Act (HIPAA) standards and implementation rules.
Once your DUA application has been received and approved, to access the data go to: http://data.healthybrainnetwork.org where you can request a LORIS account. Once you receive your access credentials:
If you experience any issues while running the query and are using Chrome, try running the query in Firefox instead. See below for a demo video:
The Document Repository stores data dictionaries for phenotypic data containing question and score labels. In order to download data dictionaries, navigate to Tools > Document Repository. On the table, navigate to Phenotypic Data Dictionaries and click on the most recent link to download a zip file containing all data dictionaries.

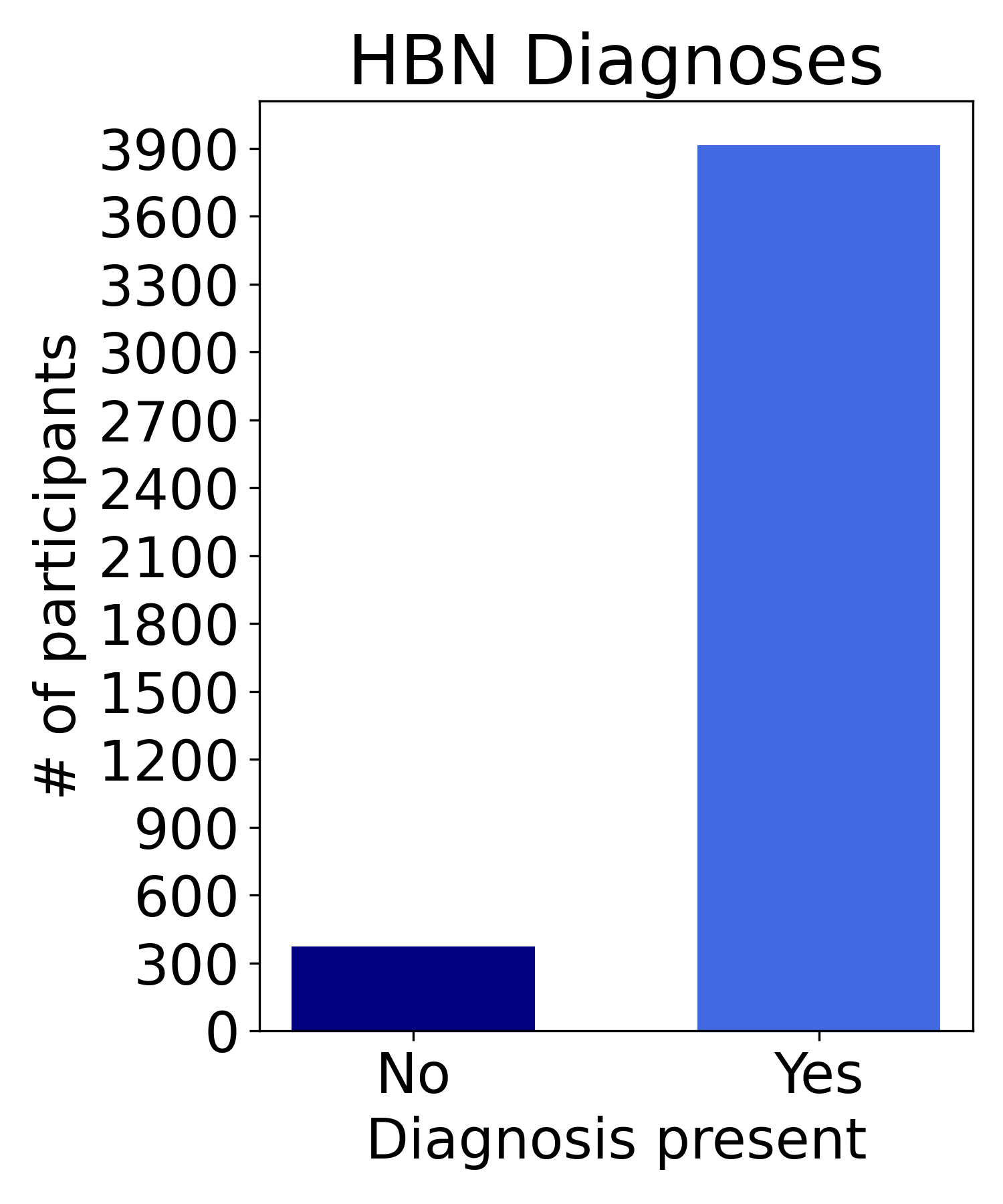
Diagnostic Process
Up to 10 diagnoses are reported in no specific order in the Diagnosis_ClinicianConsensus instrument. Diagnoses are made by a clinician, based on a combination (i.e., consensus) of different factors, including primary evidence and supporting considerations. Please note that, when communicating with prospective participants, we are careful to emphasize that our evaluation is not comprehensive for Autism Spectrum Disorder (ASD), and if their main reason for seeking the evaluation is ASD diagnosis, we refer them for a more specialized evaluation. This is because we are only able to diagnose ASD in selected cases and often it is not a confirmed diagnosis and we refer the family for further confirmatory assessment.The diagnostic process, including the specific case of ASD diagnosis, is described in details here.
Information about diagnosis (on LORIS: Diagnosis_ClinicianConsensus) is presented alongside different degrees of certainty:

- Confirmed: Full criteria for a diagnosis are met and HBN is assigning the diagnosis to the participant. HBN’s evaluation protocols are sufficient in making the diagnosis. No extra specifier is needed.
- Presumptive: Full criteria are likely met based on our evaluation and history of impairment, though HBN is unable to confirm the diagnosis due to a limitation in our evaluation protocol. The recommendations could be implemented without the need for additional testing.
- Requires Confirmation: Full criteria are likely met based on our evaluation and history of impairment, though HBN is unable to confirm the diagnosis due to a limitation in our evaluation protocol. Additionally, there is less evidence from our evaluation AND historic impairment and so less certainty than Presumptive. The disorder would require further testing in order to confirm the diagnosis. The recommendations could be implemented without the need for additional testing.
- Rule-out: Symptoms of a disorder are not clearly defined within one diagnostic criteria and/or are similar or overlap with other presenting disorders. OR Insufficient information in the HBN evaluation to make a diagnosis (or to say that the child definitively does not have a diagnosis), but concerns or vulnerabilities were evident that should be further evaluated /monitored.
- By History: A diagnosis of a disorder was reported during the HBN evaluation, though HBN is unable to confirm this diagnosis, either because the diagnosis is not fully assessed by HBN OR there was insufficient evidence on the HBN evaluation to confirm the previous diagnosis.
- Past: Full criteria for a disorder were reported during Mental Health Interview, though symptoms are reported to be no longer present for the past 2 months.
- No Diagnosis Given: The evaluation was completed and symptoms reported do not meet diagnostic criteria for any disorder.
- Incomplete Eval: The participant dropped out of the study before a diagnosis was given.